The Inflation Reduction Act (IRA) represents a landmark shift in U.S. drug pricing regulation, introducing direct federal negotiation of drug prices for the first time. Notably, the IRA imposes earlier price negotiation timelines for small molecule drugs (9 years post-approval) than for biologics (13 years).
These differential incentives may unintentionally distort the future trajectory of pharmaceutical innovation and workforce economy.
2018 research links new drug treatments to gains in labor productivity and wages among working-age adults in the United States.
Research estimates that drug innovations introduced between 2000 and 2015 resulted in 4.8 million additional work days annually (assuming 40-hour work weeks), $221 billion in annual wage gains for the U.S. working population in 2016.
Overall, drug innovation during the study period was associated with 4.8 million additional work days and $221 billion in annual wages in 2016 and $296 billion in 2025, after adjusting for inflation, with varying contributions by drug class. This research shows that medicines increase wages by $296 billion and the number of days Americans can work by 5 million each year, and that small molecule medicines, in particular, are responsible for $219 billion in wage growth annually
By further quantifying labor productivity and wage gains associated with each drug class, a new study by the HEAL Network provides a data-driven rationale for incorporating labor-sector benefits into policy design.
The chart below tracks average salary increase attributable to small molecules drugs. Also, we find small molecule drugs provide more consistent productivity in terms of hours worked per year when compared to biologics.
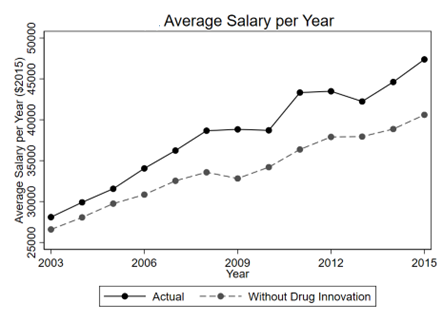
Small molecule drugs, more commonly used across widespread chronic and mental health conditions, contributed to broader and more evenly distributed gains across the working population.
These results have critical implications for the evaluation of pharmaceutical value and the design of drug pricing policy—particularly in light of the Inflation Reduction Act (IRA). The IRA introduces a mechanism for Medicare to negotiate drug prices, starting in 2026, with different time thresholds for biologics (13 years post-approval) and small molecule drugs (9 years post-approval). While designed to curb excessive drug spending, these provisions may unintentionally distort future innovation incentives.
Differential Policy Effects on Innovation Incentives
The earlier negotiation timeline for small molecule drugs may disincentivize investment in therapeutic areas with high population burden but moderate pricing power—precisely the conditions under which many of the productivity-enhancing small molecule drugs operate. Mental health, metabolic disorders, and musculoskeletal conditions—all associated with major economic losses from absenteeism and presenteeism—may see reduced future investment if manufacturers perceive a compressed revenue horizon.
Labor Productivity: A Missing Piece in Drug Value Assessment
Current frameworks for cost-effectiveness and value-based pricing rarely include downstream economic returns from improved labor participation. This omission is critical. Our analysis estimates that over $221 billion in annual wage gains and nearly 5 million additional workdays per year attributable to drug innovation—figures that dwarf traditional health care savings and rival broader macroeconomic policy impacts.
Implications for Policy and Practice
Reform IRA Implementation: To avoid stifling innovation in high-productivity-impact conditions, policymakers should consider adjusting the IRA’s timelines or providing exemptions for drugs that show quantifiable labor market returns—especially for small molecule innovations treating prevalent, economically disruptive diseases.
Conclusion
The Inflation Reduction Act, while a landmark in drug pricing reform, introduces differential negotiation timelines that may unintentionally penalize innovation in high-prevalence therapeutic areas. If pricing pressure disproportionately affects small molecule therapies, the long-term societal benefits of improved work ability—especially in economically vulnerable populations—may be diminished.
Our findings reinforce the need for a broader, more holistic approach to drug valuation and policy design. Labor market returns, such as increased hours worked and higher earnings, represent a major share of the total value created by pharmaceutical advancements. These productivity gains should be formally incorporated into health technology assessments, cost-effectiveness frameworks, and pricing negotiations to ensure that innovation is aligned with the full spectrum of societal benefit.
As health systems and policymakers continue to grapple with rising drug costs and sustainability, it is essential to preserve incentives for therapies that restore not only health, but also human productivity and economic independence. Failing to do so risks undervaluing innovation that empowers patients to participate fully in work and life.
Provisions in the IRA, particularly differential negotiation timelines for drug classes, may inadvertently shift innovation incentives in ways that undermine societal productivity gains.